The pharma options can be downloaded here as a datasheet PDF.
Option 1: Verification Kit

Used for the regular monitoring of the measuring accuracy of the IPP probe. To this end, the probe is clamped to the verification kit and 3 calibration pins rotate through the measuring volume. The calibration pins correspond precisely to particle sizes 150, 1000 and 2000 µm.
All necessary documents for the installation qualification (IQ) and operational qualification (OQ) of the IPP probes are provided.
Option 2: polished contact surfaces

Contains polished stainless steel surfaces (316L) for all of the surfaces of IPP probes that will come into contact with the product as well as of all auxiliary parts, such as the disperser, including comprehensive documentation and 3.1 certificates that ensure the traceability of the materials used.
Option 3: Parsum View
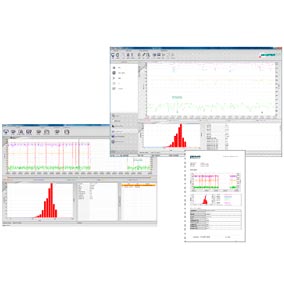 This is a fully independent user interface for the monitoring, control and measured-data handling of IPP probes. Thanks to the measuring software communication via TCP/IP protocol, the interface can be used locally or within a network. Operation is quick and intuitive thanks to ergonomic menus and a large and variable data display.
This is a fully independent user interface for the monitoring, control and measured-data handling of IPP probes. Thanks to the measuring software communication via TCP/IP protocol, the interface can be used locally or within a network. Operation is quick and intuitive thanks to ergonomic menus and a large and variable data display.
This software was specifically designed for areas with pharmaceutical requirements (e.g., with regard to 21 CFR Part 11). Standard software packages (Microsoft SQL server) and Parsum software ensure that this is an efficient and reliable solution.
The software has the following features:
- Detailed access monitoring
- User management
- Audit trail for monitoring and saving all accesses and changes
- Secure storage of all data and settings in an SQL database
